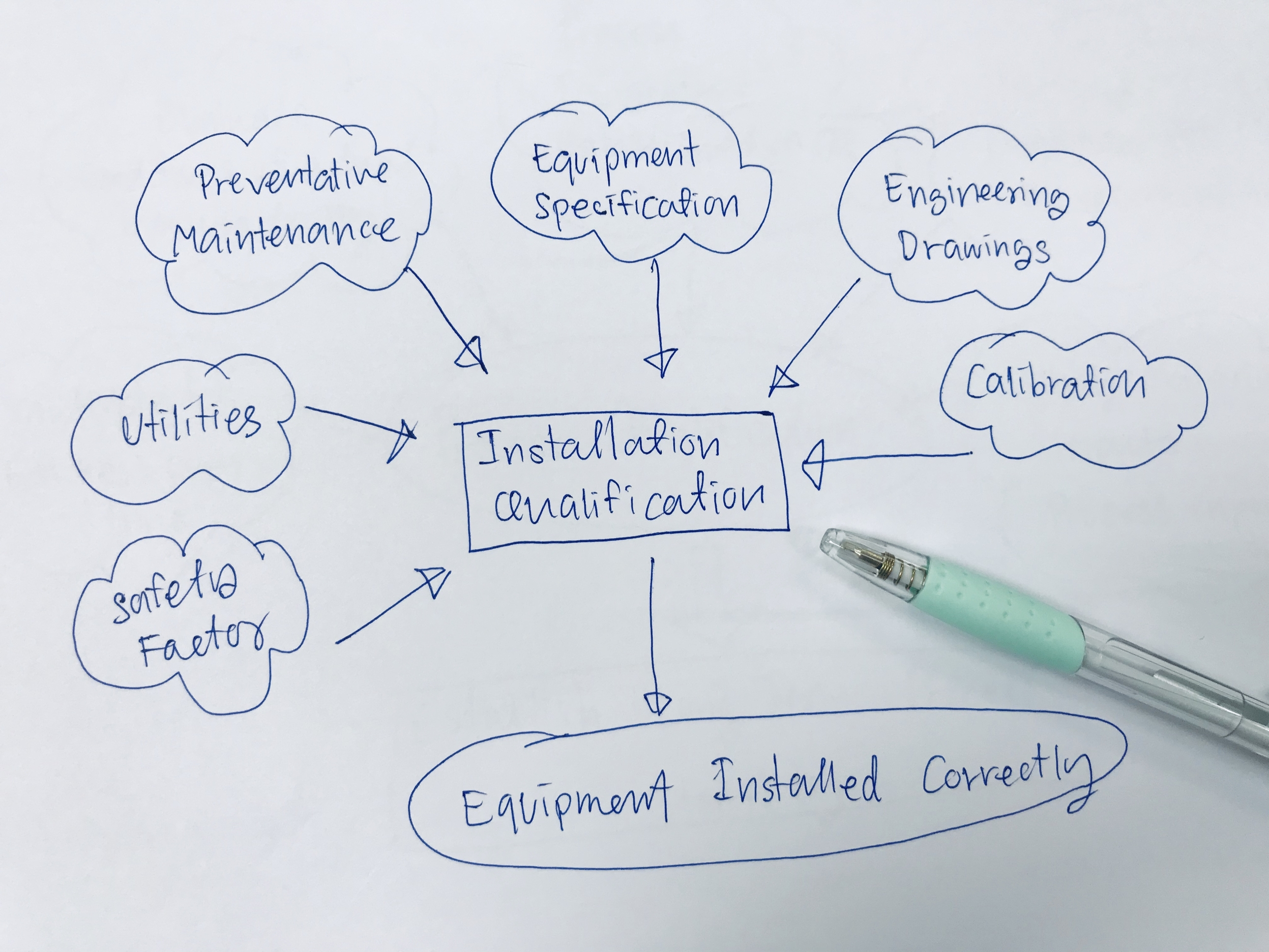
What is Installation Operational Qualification (IOQ)?
In pharmaceutical, biotechnology, and laboratory environments, precision isn’t just expected—it’s required. Every piece of equipment, system, or cleanroom space must be installed correctly and function as intended before it can be used for regulated activities. That’s where installation operational qualification (IOQ) is essential. IOQ is a combined validation protocol used to verify that laboratory and […]
 Small But Mighty
Small But Mighty